 .,,
.,,
A01 Air can be compressed and expanded
 .,,
.,,
Take an "empty" plastic bottle
and close it tightly. Try to crumble the bottle by standing on it. Observation:
The
pressure of your body is not sufficient to crumble the bottle as it is
full of air.
By
pumping air into the inner tube of your bicycle you know that air can
be compressed. Left: This closed bottle was photographed
20
m under water by a scuba diver. It is extremely compressed!
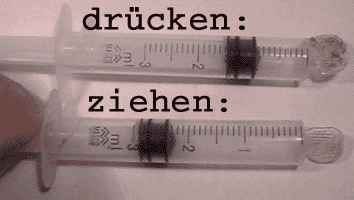
Right: These two
3-ml syringes contained 2 ml of air, when their openings were closed by
welding.
Observation:
The
air locked in the syringe can be compressed and expanded by pushing
and pulling the piston. Conclusion:
If
you measure the volume of air you
also have to know its pressure.