

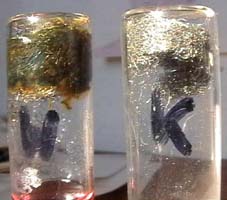
Iron rusts at the air and
a new substance is formed. Test what happens with the air during rusting.
What
you need: 4 Liquemin
bottles, 2 stoppers pierced by drinking straws, red water, two pieces
of steel wool.
* Left: Press steel
wool on the bottom of two bottles. * Moisten the steel wool in the bottle
marked W, the other one keep dry for control (K).
* Close the two bottles,
push the two tubes only slightly into the stoppers, * Dip them into bottles
full of red water.
* Observations
middle: The water only climbs
up into the bottle with moistend steel wool. 1/5 of it contains water in
the end. * Right: Only the moistened steel wool
looks rusty brown.
Explanation:
The iron of the steel wool made
a chemical reaction with oxygen of the
air and water. As oxgen disappeared during this reaction the volume of the
air decreased inside the bottle.