Microscale Chemistry Experimentation for Teachers

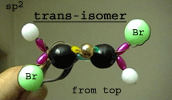
24.
C2H2Br2 orbital model (sp2
hybridization): cis-trans-isomerismDuring
the reaction between
bromine water and acetylene
(ethine) two isomers of 1,2-dibromoethene were obtained.
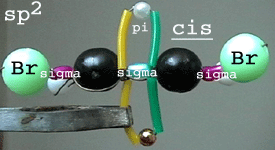
Left: cis-1,2-dibromoethene
isomer showing the sigma skeleton from side and one p orbital (pi
bond) above and under the planar molecule. Both Br atoms can
be seen in front of the sigma skeleton.
Photo 2: The same
molecule model showing the complete sigma skeleton from the top.
The model of the cis
isomer shows that the two Br atoms are on the same side
of the sigma skeleton.
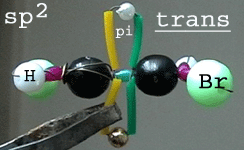
Photo 3: trans-1,2-dibromoethene
isomer showing the sigma skeleton from side and one p orbital (pi
bond) above and under the planar molecule. Only one of the two Br atoms
is in front of the sigma skeleton.
Right: The same molecule
model showing the complete sigma skeleton from the top.
The model of the trans
isomer shows that the two Br atoms are on different sides of
the sigma skeleton.
The molecules of these two stereoismers are visualized by assembling white, black and green beads (models of H, C, Br atoms) long microbeads (sigma bonds), round microbeads and plastic tubings ( pi bonds) by wire.