Microscale Chemistry Experimentation for Teachers
 ................................
................................
29.
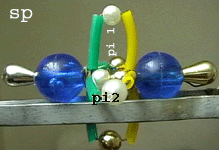
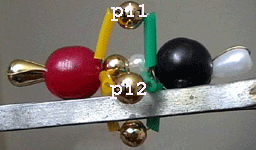
Tripple bonding in nitrogen and carbon monoxide molecules
(sp hybridization)
* All atoms are sp hybridized
(sigma bond).
* The tripple
bond consist of one sigma and
two pi bonds, oriented vertically
to the nuclear axis.
* One of the pi
bonds in the CO molecule is a dative bond with
the O atom contributing both bonding electrons.
* Two non-bonding electrons
from each atom constitute one non-bonding pair
of electrons (pear-shaped micro beads).