 .
. .
. Electrolysis
of sodium sulfate solution in a 5-mL-bottle and in a blister
Take safety precautions concerned with electricity. Work on a tray
Left:
Puncture the stopper of a Liquemin bottle
by two hypodermic needles, blunt their tips. Fill the bottle with conc.
sodium sulfate solution and close it by the stopper.
Electrolysis
of sodium sulfate solution in a 5-mL-bottle and in a blister
Take safety precautions concerned with electricity. Work on a tray
Left:
Puncture the stopper of a Liquemin bottle
by two hypodermic needles, blunt their tips. Fill the bottle with conc.
sodium sulfate solution and close it by the stopper.
Connect the needles
with a 9-Volt battery. Place the bottle upwards down on a second bottle.
Observations:
* Gas bubbles
appear at both electrodes, more bubbles can be seen at the cathode. The liquid
is pressed out of the upper bottle through the blunted needles.
* The gas explodes
when lit in a candle.
Middle:
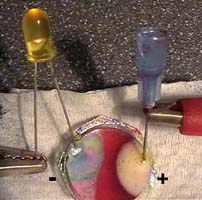
Build an electric circuit by a 9-Volt battery, two insulated wires with crocodile
clips, a light emitting diode (LED) and a blunted needle both dipped into
a blister with sodium sulfate solution + red cabbage indicator.
Observations :
Right:
Gas bubbles appear at both electrodes, more bubbles can be seen at the cathode
(-). The colour of the indicator around the cathode
changes to green/blue.
Explanation:
Red: 4 H2O(l) + 4 e- --> 2 H2(g)
+ 4 OH-(aq)
Ox:
2 H 2O(l) --> O2(g) +
4 H+(aq) + 4 e -
Redox:
6 H 2O(l) --> 2 H2 (g) + O2
(g)
+ 4 OH-(aq) + 4 H+(aq)
Colour change
: The different colours of the red cabbage juice
result from acid base reactions of coloured compounds called anthocyans (anthos
= flower, kyanos = blue). Their cations "are especially interesting because
they behave much like the pH indicator..." (S.S.Zumdahl, Chemistry) phenolphthalein.
back
.
go on
....
contact Hugerat
........,,.
contact Schwarz
.............................................
last modification: 18.03.2002