 .
. .
. 4.
Testing
the quality of distillation: Measuring electric conductivity
(Makrochemie:
Chemie heute S1, 2001, S.18)
4.
Testing
the quality of distillation: Measuring electric conductivity
(Makrochemie:
Chemie heute S1, 2001, S.18)
Electricity
(current) is something that comes
out of a battery or out of a socket and makes light in a bulb. An
electric
circuitis needed to make electricity flow.
Never
ever take electricity from a bulb for your experiments: THIS CAN BE DEADLY!
What
you need
A piece of tin and a closure of
a cola can made of steel (test by a magnet), insulated copper wire, 4 crodile
clips, a meter for electricity, 9-Volt battery Light-emitting
Diode(LED),
blister
for salt water, Cola and tap water, a small pencil which is sharpened on
both sides.
Experiment
1 (Left):
Make
a "battery" from the two different metals of a Cola can.
* Connect the closure of
a Cola can with the negative terminal of your multimeter (COM).
* Clean a piece of Cola
by sandpaper and connect it with the positive terminal (red). Switch in
the meter: 20 V
* Press the two metals between
your thumb and forefinger. The meter reads 0.24 Volt.
Later
on you will repeat such experiments to learn about batteries.
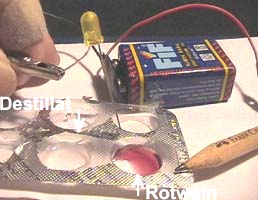
Experiment
2 (Middle and right):
Test
red wine and its distillate using the battery and the LED
*
Connect the negative
terminal of your 9-Volt battery with the short pin of the LED.
* The positive terminal
of the battery is connected with one end of the pencil.
* Dip the other end of the
pencil and the long pin of the LED into the blister with 1 ml of sea water.
Observations
and Explanations
* Middle: The LED
shows no light in the distillate of red wine: Distilled water has nearly
no electric conductivity.
.............(The
LED shows light because: Red wine conducts electricity fairly well).
* Right: (After adding
a few grains of table salt to a drop of the distilled water) The LED shows
light / bubbles / a smell like in the swimming pool:.............
.............You
have done your first chemical experiment:
Electric
current transformed salt into chlorine. This is called
electrolysis.
back................go
on.........,,.............................,,,,,,,,,...........
...... .....................last
modification: 24.04.2002
 .
. .
.
 .
. .
.