 .
. .
. .
.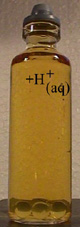 .
. .
.
wat14 Measuring
the O2 concentration of water ("Mini-Winkler")
Plants
and animals in
freshwater, in sea
water need oxygen to survive.
Water
dissolves only small amount of oxygen, its solubility decreases dramatically
when the temperature increases.
Photo
1: AquamerckR Kit to determine
oxygen in 50 ml samples using the Winkler method .
In
Photo 2 - 5 you can see the consecutive steps using Liquemin
injections bottles:
2)
Fill a vial with water using a 20-ml syringe with a long needle.
Start at the bottom and prevent air bubbles.
3)
By adding 2 drops of solution containing Mn2+, OH-
and I- ions a brown precipitate is formed. Keep the vial.4)
Add sulfuric acid (H+aq) to release a quantitity of iodine which
is equivalent to the quantity of O2 in the sample.
5)
Titrate the iodine dissolved in 5-ml of solution by a sodium thiosulfate
solution using a starch solution as an indicator.

.
The
volume of thiosulfate solution needed to change the colour of the sample
from blue to colourless is a measure for the oxygen content of the water.
last
photo: Digital display of the O2 content to control the
iodometric method.
back.......
go
on.........................................................................................................last
modification: 25.10.2001