 .
. .
. ..
..
8.
Heat
at work / Candle as "steam engine"
Energy is "the
capacity to do work or to cause a heat flow". (Zumdahl, Chemistry)
Work is a force over
a distance. Force is everything "pushing, pulling and lifting"(P.Warren,
Physics for life).
What
you need: tray with water, candle as burner, micro spirit burner
from a Liquemin bottle, matches, tissue paper, Liquemin bottle with a stopper,
"test tube holder": 15 cm insulated copper wire (2 x 0,6 mm), methylated
spirit.
Experiment
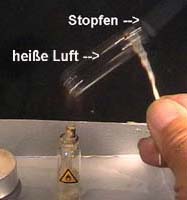
1 (Photo 1):
Heat locked air
by a candle and by a micro spirit burner
1.
Stopper an "empty" Liquemin bottle.
2.
Twist the copper wire around it as a "test tube holder".
Photo
1: Heat the bottle by the micro burner and after that by the
candle. Observations?
You
should learn from experiment 1:
The
heat energy released by the chemical
reaction of the spirit burner was transferred to the air locked in
the bottle.
A
pressure was produced inside the bottle. Photo 1: The pressure
carried out work on the stopper transferring kinetic energy on it.
The
heat energy released by the chemical
reaction of the candle was not sufficient to move the stopper.
Wax
was transformed into soot.
Experiment
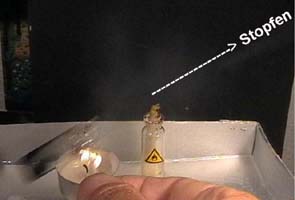
2 (Photo 2, 3):
Heat locked water
drops by a candle
1.
Heat water drops on the bottom of a closed Liquemin bottle
by a candle.
2.
Heat the upper part of the bottle. (Photo 2)
Photo
3: Observe,
what happens with the stopper, the bottle and the flame.
You
should learn from experiment:
Water
drops are evaporated by the heat of the candle. Water vapour condensed
in the upper part of the bottle.
The
water vapour produced by the candle light carried out work on the stopper:
It
moves forward, while the vial is pushed into the opposite direction.
back
go on..........................................................................................last
modification: 31.10.2002