Microscale Chemistry Experimentation for Teachers
 ....
.... ....
....
23.
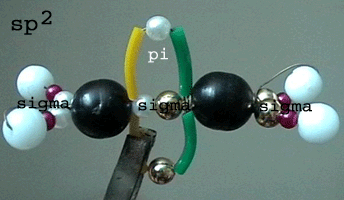
C2H4 orbital model (sp2 hybridization)
Left Model
of a sp2
hybridized C atom
(black bead): three orbitals (golden microbeads) arranged
at 120° angles in the same plane.
A fourth (unhybridized)
p-orbital (not modelled) is oriented perpendicular to this plane: Above
and below the black bead.
Middle: Molecule
model of ethen (basic version).
Each C atom (black bead)
forms 3 sigma bonds with
2 H-atoms and 1 H-atom (white bead) at angles of 120° pointing to the
three corners of a triangle. The fourth bond is a second C-C bond called
pi
bond (long pink bead). So the two C atoms in the ethene molecule
have a double bond.
Right By modelling
the ethylen molecule this way you can learn more about the molecular structure
and the chemical properties:
The skeleton of the ethylen
(C2H4) molecule is made
up by 5 sigma bonds between the 6 atoms.
Each sigma bond is assembled
by a pair of microbeads.
The green-yellow plastic
loop which is perpendicular to the plane of the sigma skeleton is the model
of the pi bond formed by the overlap of the fourth unhybridized orbital
of each C atom. The structure of this pi molecular orbital is the reason
for the high reactivity of ethylen.
The molecule model of C2H4
resembles that of oxygen.