Microscale Chemistry Experimentation for Teachers
 ..
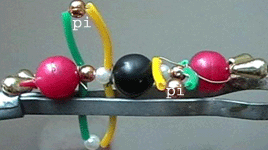
..
26.
Double bonding in the O2 and CO2 molecules
The double bonds consist
of
* one sigma
bond formed by overlap of two hybrid orbitals (the two adjacent
white and golden micro beads) and
* one pi
bond vertically oriented to the sigma bond axis and formed by the
lateral overlap of two unhybridized p atomic
orbitals (the plastic
loop coloured green/yellow, with two micro beads representing bonding electrons
contributed one from
each O and C atom
and present above and below the sigma internuclear axis).
(compare
CO molecule model).*