Microscale Chemistry Experimentation for Teachers
28.Nitrogen
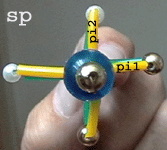
(sp hybridization)
Each of the two N atoms
(blue beads) has 2 sp hybrid orbitals represented
by one round and one pear-shaped microbead. They point
away from each other forming a streight line (sigma skeleton).
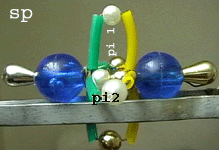
Left:
The nitrogen molecule model showing the sigma skeleton seen from the side:
* One white micro bead of
each N atom forms the sigma bond (overlap
of 1 of the sp hybrid orbitals).
* The 2. sp hybrid
orbital is occupied by two lone pairs electrons.
* Two N-N pi
bonds are modelled by two plastic loops.
* Right: In
the same model the green and yellow plastic loops can be better seen because
the line points to the observer.
* The loops represent the
2N-N
pi
bonds formed by the overlap of the two unhybridized p orbitals
of each N atom.
* They are perpendicular
to the sigma skeleton.
* It can be seen that one
of the two pi bonds is oriented horizontally and the other one vertically.
1 N-N
sigma
bond and 2 N-N pi bonds
form a tripple bond.