(Page under construction)
*) Dr.
Muhamad Hugerat , Arab College for Education in Israel has used
this title for his book for children.
 .....
.....  .....
..... 
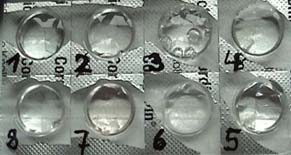
Materials
Fig. 1 - 2: Tray,
re-usable tablet container with 8 blisters, battery water (1), fresh
water (2), soda water (3), water with table salt (4), water with Dead Sea salt (5), water with Kaiser
Natron (6), water with washing
soda (7), lime water (8),
9-Volt battery, two insulated copper wires with crocodile clips, light
emitting diode (LED), pencil lead, spatula
(cut from a drinking straw)
, plastic pipette, stirring
rod (piece of insulated copper wire), tissue paper.
Experiment
1. Add spatula tips
of table salt, Dead Sea Salt, Kaiser Natron and washing soda to blisters
4, 5, 6 and 7.
2. Starting with battery water add 10 drops to blister 1, 4, 5, 6 and
7.
3. Put 10 drops of fresh water into blister 2, 10 drops of soda water
into blister 3 and 10 drops of lime water into blister 8.
4. Stir in blisters 4 - 7 to dissolve the salts. Clean the stirring wire
after each use.
5. Connect the positive terminal of the battery with the pencil lead and
the negative terminal with the short wire of the LED.
6. Starting with blister 1, close the circuit by dipping the free ends
(wire and lead) into the liquid.
7. Go on with blisters 2, 3, 8, 4, 5, 6, 7.
Observations
1. The LED show light of different
brightness in blisters 2 - 8. See Fig. 3 showing blister 4.
2. In blister 1 no light at all can be seen.
Explanation
1. Die Leitung des
Stroms durch Flüssigkeit besorgen positiv und negativ geladenene Teilchen
(Ionen).
Je mehr davon gelöst sind, desto mehr Strom fließt,
und desto heller leuchtet die LED
2. Auch in Blister 1 sind Ionen vorhanden. Der von ihnen ermöglichte
Stromfluss lässt sich nicht durch die LED testen, wohl aber durch ein
hochohmiges Messgerät registrieren.
Addition
See experiment 3.3
To prevent chemical reactions during measuring electric conductivity
alternative current is needed.
back.......................................................................................................................... last
modification: 27.02.2006